

Journal of Geographical Sciences >
Spatial-temporal patterns of major ion chemistry and its controlling factors in the Manasarovar Basin, Tibet
*Corresponding author: Wang Rui (1987-), PhD Candidate, E-mail:wangr.12b@igsnrr.ac.cn
Author: Yao Zhijun (1959-), Professor, specialized in hydrology and water resources studies. E-mail:yaozj@igsnrr.ac.cn
Received date: 2014-11-17
Accepted date: 2015-01-22
Online published: 2015-06-15
Supported by
National Natural Science Foundation of China, No.41190080
“Strategic Priority Research Program (B)” of the Chinese Academy of Sciences, No.XDB03030400
Copyright
The Manasarovar Basin in southern Tibet, which is considered a holy land in Buddhism, has drawn international academic attention because of its unique geographical environment. In this study, based on actual measurements of major ion concentrations in 43 water samples collected during the years 2005 and 2012, we analyzed systemically the spatial- temporal patterns of water chemistry and its controlling factors in the lake and inflowing rivers. The results reveal that the water in the Manasarovar Basin is slightly alkaline, with a pH ranging between 7.4-7.9. The amounts of total dissolved solids (TDS) in lake and river waters are approximately 325.4 and 88.7 mg/l, respectively, lower than that in most of the surface waters in the Tibetan Plateau. Because of the long-term effect of evaporative crystallization, in the lake, Na+ and HCO3- have the highest concentrations, accounting for 46.8% and 86.8% of the total cation and anion content. However, in the inflowing rivers, the dominant ions are Ca2+ and HCO3-, accounting for 59.6% and 75.4% of the total cation and anion content. The water exchange is insufficient for such a large lake, resulting in a remarkable spatial variation of ion composition. There are several large inflowing rivers on the north side of the lake, in which the ion concentrations are significantly higher than that on the other side of the lake, with a TDS of 468.9 and 254.9 mg/l, respectively. Under the influence of complicated surroundings, the spatial variations in water chemistry are even more significant in the rivers, with upstreams exhibiting a higher ionic content. The molar ratio between (Ca2++Mg2+) and (Na++K+) is much higher than 1.0, revealing that the main source of ions in the waters is carbonate weathering. Although natural processes, such as rock weathering, are the major factors controlling main ion chemistry in the basin, in the future we need to pay more attention to the anthropogenic influence.
YAO Zhijun , WANG Rui , LIU Zhaofei , WU Shanshan , JIANG Liguang . Spatial-temporal patterns of major ion chemistry and its controlling factors in the Manasarovar Basin, Tibet[J]. Journal of Geographical Sciences, 2015 , 25(6) : 687 -700 . DOI: 10.1007/s11442-015-1196-5
Figure 1 Drainage system of the Manasarovar Basin and the sampling sites |
Table 1 Average content of the main chemical components of waters in the Manasarovar Basin |
| K+ | Ca2+ | Na+ | Mg2+ | Cl- | SO42- | HCO3- | SiO2 | TDS | pH | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2005 measurements | ||||||||||
| Lake | 4.76 | 35.72 | 49.54 | 32.68 | 14.03 | 33.24 | 347.54 | - | 343.7 | - |
| River estuaries | 0.84 | 16.47 | 4.70 | 4.18 | 1.68 | 23.69 | 52.28 | - | 77.7 | - |
| Basin | 2.80 | 26.10 | 27.12 | 18.43 | 7.85 | 28.47 | 199.91 | - | 210.7 | - |
| 2012 measurements | ||||||||||
| Lake | 6.24 | 20.11 | 54.23 | 29.28 | 14.57 | 31.03 | 299.30 | 1.87 | 307.0 | 7.85 |
| River estuaries | 1.68 | 18.65 | 7.02 | 4.75 | 2.10 | 18.14 | 75.59 | 9.56 | 99.7 | 7.78 |
| Upper rivers | 1.19 | 23.64 | 14.69 | 6.95 | 7.95 | 15.08 | 115.42 | 7.88 | 135.1 | 7.42 |
| Basin | 3.04 | 20.80 | 25.31 | 13.66 | 8.21 | 21.42 | 163.44 | 6.44 | 180.6 | 7.68 |
| Average levels between 2005-2012 measurements | ||||||||||
| Lake | 5.50 | 27.91 | 51.89 | 30.98 | 14.30 | 32.14 | 323.42 | - | 325.36 | - |
| River estuaries | 1.26 | 17.56 | 5.86 | 4.46 | 1.89 | 20.92 | 63.94 | - | 88.70 | - |
| Precipitation | 2.66 | 10.54 | 3.19 | 1.36 | 4.16 | 9.27 | 32.76 | - | 47.56 | - |
| Basin | 2.92 | 23.45 | 26.22 | 16.04 | 8.03 | 24.94 | 181.67 | - | 195.65 | - |
Note: the units of TDS and ions concentration are mg/l. |
Table 2 Comparison of chemical components for lakes in the Tibetan Plateau |
| Lake | TDS | K+ | Na+ | Ca2+ | Mg2+ | Cl- | SO42- | HCO3- | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Manasarovar | 0.31 | 0.14 | 2.31 | 0.61 | 1.27 | 0.42 | 0.34 | 5.11 | This study |
| Qinghai | 18.05 | 4.24 | 191.8 | 0.57 | 33.44 | 226.4 | 29.52 | 29.16 | Xu et al., 2010 |
| Nam Co | 1.2 | 0.89 | 13.91 | 0.18 | 3.28 | 3.65 | 2.15 | 13.8 | Wang et al., 2010b |
| Tangra Yumco | 11.1 | - | - | - | - | - | - | - | Wang et al., 2010a |
| Zhari Namco | 7.74 | - | - | - | - | - | - | - | Wang et al., 2010a |
| Pumayum Co | 0.1 | 0.45 | - | 1.12 | 1.03 | 0.05 | 0.81 | 1.75 | Ju et al., 2010 |
| Yamzhog Yumco | 1.28 | 0.46 | 12.29 | 0.18 | 7.85 | 2.31 | 7.25 | 9.74 | Sun et al., 2013 |
| La'ang Co | 0.49 | 0.52 | 7.65 | 0.32 | 4.48 | 1.94 | 1.12 | 13.34 | Wang et al., 2013 |
Note: The unit of TDS concentration is g/l, and the units of main ions concentration are mmol/l. |
Figure 2 Ternary plots of (a) cations and (b) anions of the Lake Manasarovar, its inflowing rivers, and rain samples |
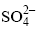 are evaporites, it can be deduced that the processes of chemical weathering of evaporites are on the rise. By contrast, the effect of carbonate and silicate weathering, which mainly contributes to the HCO3- in waters, is on a decreasing trend.
are evaporites, it can be deduced that the processes of chemical weathering of evaporites are on the rise. By contrast, the effect of carbonate and silicate weathering, which mainly contributes to the HCO3- in waters, is on a decreasing trend. , were slightly lower in 2005, and then increased significantly in 2012, with a rate between 30.4% (Ca2+) and 185.0% (Na+). During 2005-2012, the concentrations of most of the ions in the basin river waters increased dramatically, with an average TDS amplification of 51.8%, and a rate of increase for each ion ranging between 30.3% (Ca2+) and 124.8% (Na+).
, were slightly lower in 2005, and then increased significantly in 2012, with a rate between 30.4% (Ca2+) and 185.0% (Na+). During 2005-2012, the concentrations of most of the ions in the basin river waters increased dramatically, with an average TDS amplification of 51.8%, and a rate of increase for each ion ranging between 30.3% (Ca2+) and 124.8% (Na+).Table 3 Main ions concentration in the Manasarovar Bain during different periods (mg/l) |
| K+ | Ca2+ | Na+ | Mg2+ | Cl- | SO42- | HCO3- | TDS | |
|---|---|---|---|---|---|---|---|---|
| Lake center | ||||||||
| 1976a | 5.92 | 24.31 | 44.05 | 25.72 | 14.83 | 26.3 | 264.66 | 277.04 |
| 2009-2010b | 5.97 | 26.86 | 49.09 | 28.73 | 13.83 | 29.73 | 300.71 | 304.57 |
| Lake peripheries (L1 sample for 2005 and 2012) | ||||||||
| 1976a | 3.23 | 22.13 | 24.21 | 16.35 | 9.6 | 15.75 | 178.03 | 182.88 |
| 2005 | 6.32 | 69.55 | 59.30 | 41.73 | 10.21 | 13.93 | 556.17 | 479.12 |
| 2012 | 7.75 | 35.08 | 65.21 | 36.35 | 14.62 | 28.41 | 415.61 | 395.22 |
| River estuaries (R5 sample for 2005 and 2012) | ||||||||
| 1976a | 1.65 | 16.13 | 6.35 | 5.33 | 3.68 | 8.06 | 67.69 | 80.60 |
| 2005 | 0.81 | 24.17 | 4.13 | 3.59 | 1.69 | 36.34 | 55.10 | 98.28 |
| 2012 | 1.17 | 31.50 | 11.77 | 8.77 | 4.31 | 15.88 | 146.12 | 146.46 |
Note: a the data is from the reference of Guan et al. (1984); b the data is from the reference of Wang et al. (2013) |
Figure 3 Concentration (meq/l) of (a) cations and (b) anions in different water samples of the Lake Manasarovar, showing the Cl- and content expanded by five times |
Figure 4 Equivalent (meq/l) of (a) cations and (b) anions in water samples of rivers in the Manasarovar Basin, showing the Cl- and SO42- content expanded by five and three times, respectively |
Figure 5 The Gibbs plot of the Lake Manasarovar and its flowing rivers |
Table 4 Contribution magnitude and percentage of main ions from atmospheric inputs to the river waters in the Manasarovar Basin |
| K+ | Ca2+ | Na+ | Mg2+ | SO42- | HCO3- | Cl- | |
|---|---|---|---|---|---|---|---|
| Magnitude (μmol) | 4.90 | 19.93 | 11.01 | 4.19 | 7.20 | 40.64 | 9.11 |
| Max. percentage (%) | 37.8 | 21.2 | 35.4 | 22.8 | 24.9 | 81.2 | 100.0 |
| Mean percentage (%) | 21.1 | 7.1 | 9.4 | 5.7 | 6.7 | 10.8 | 47.5 |
| Min. percentage (%) | 3.5 | 1.9 | 0.7 | 0.6 | 1.3 | 1.3 | 1.3 |
Figure 6 Equivalent comparison between different ions in the study area, showing (a) Na+ vs. Cl-, (b) Na++K+ vs. Ca2++Mg2+, (c) Ca2+ vs. , (d) Ca2++Mg2+ vs. |
The authors have declared that no competing interests exist.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
/
| 〈 |
|
〉 |